
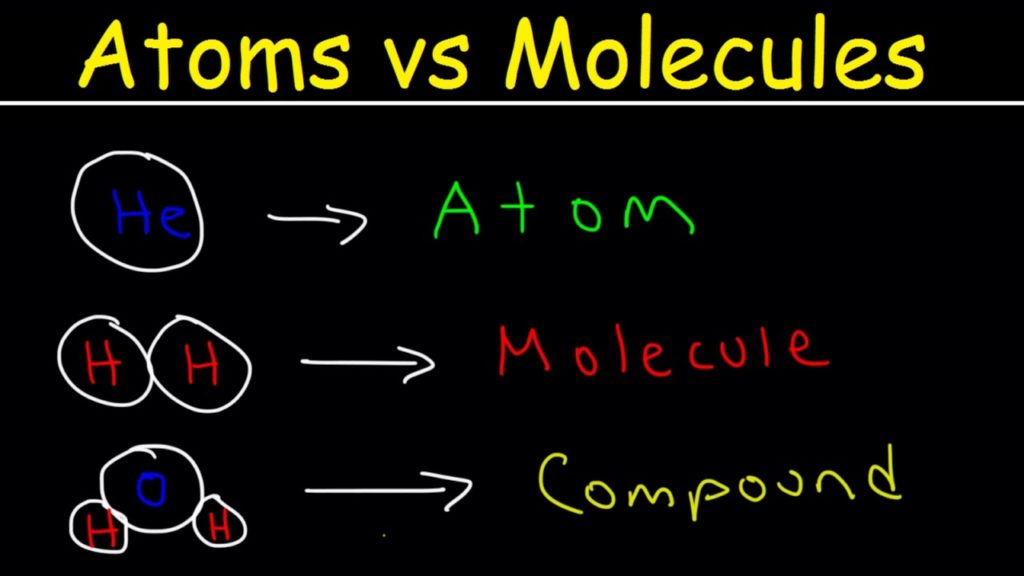
The nucleus of an atom is composed of protons and neutrons within the center of the atom. Atoms are composed three subatomic particles: protons, neutrons and electrons (fig. The fundamental unit of matter is the atom, meaning this is the smallest particle of matter that has the properties of that substance. Matter is defined as physical substances, which contain mass and take up space. Water is a great example of just 3 atoms (H, O, and H) held together with 2 bonds (two identical H‑O bonds).Chemistry is the study of matter. A true molecule has a beginning and an end - held together with covalent bonds. We call these repeat units in ionic compounds formula units. That is why sodium chloride is just NaCl. Even though the entire cluster/lattice is all interconnected with ionic bonds, we only show the basic repeat unit in formulas. When true positive ions and negative ions get together, they create an entire lattice of alternating positive and negative charges (cations and anions to be specific). There is one more specific thing I'd like to point out. So know that there are special cases of elements as molecules. So iron in a reaction scheme is just Fe(s) if it is in the solid state. However, most of the elements are depicted in reactions as just the single symbol for that element. There are other molecular elements as well.
#Atom vs molecule plus
Those are both diatomic molecules AND they are elements - plus they match up well with the little purple diatomic molecule shown.

Like the two examples of nitrogen and oxygen I mentioned earlier. So are all compounds molecules? and all elements are atoms? No, I just pointed out that some elements can be made up of molecules.Īs pointed out on the figure, certain elements exist as molecules and not atoms. Also note that two of the molecules (the two diatomic ones) are elements (not compounds) because the molecule is made out of only one kind of atom which means it's an element. Water, H 2O, is a compound - and a molecule. A compound is any substance made up of more than one type of atom (element) - which means there are only two compounds shown in the figure above - the two on the far right end which each have 3 atoms in them. There are two simple diatomic molecules (2 atoms) while the others shown have three and four atoms.Īlso, don't get confused with atom/molecule and element/compound either. The molecules are all combinations of atoms in some way. The range of atom sizes actually shows the entire relative range of atom sizes from the smallest (hydrogen) to the largest (cesium). The circles actually represent a three dimensional sphere. A molecule is the simplest repeat unit of most of matter. Some molecules in your body are incredibly large consisting of thousands of bound atoms with molar masses in the hundred thousand range. Those are very small molecules relatively speaking. The air you are breathing has two major diatomic molecules, nitrogen gas and oxygen gas. In this case, a diatomic molecule (prefix di- means two). Two atoms that are bound together via a chemical bond make a molecule. Molecules need a combination of atoms to be complete. Neutrons vary but will typically be the same as the number of protons (smaller atoms) or 1.5 times the number of protons (larger atoms). All neutral atoms (no charge) have the exact same number of electrons as they have protons. Gold is an atom with 79 protons in the nucleus. 6 protons in the nucleus is a carbon atom. The number of protons is the key to which element the atom is. And, although atoms are built from the same subatomic particles (protons, neutrons, and electrons), the elements are uniquely different in their properties in that each atom is built with a different number of protons, neutrons, and electrons. An iron atom is different from a carbon atom. The name of the atom is the element name. For now though, we shall leave the atom intact. Those events like that fit into a category called nuclear chemistry and we will touch on it eventually. The atom is very very robust and stays that atom forever unless some extraordinary event of high energy occurs. Standard chemistry and chemical reactions do NOT break atoms into smaller pieces. Think of atoms as our fundamental building block for all matter. The elements on the periodic table match up one-to-one for each type of atom there is in the universe as we know it. Atoms are the very simplest units of matter that are uniquely different for each element.


 0 kommentar(er)
0 kommentar(er)
